What is MBTA Immunotherapy?
MBTA immunotherapy is a new type of cancer treatment that combines the effects of:
- Mannan – a substance derived from yeast cell walls that stimulates innate immunity,
- BAM – a bifunctional antibody molecule that binds tumor antigens and simultaneously attracts immune system components,
- Toll-like receptor agonists – molecules that act as alarm signals for the immune system, further enhancing the immune response.
This combination triggers a powerful and coordinated response from both innate and adaptive immunity.
Unlike standard chemotherapy or radiotherapy, which typically destroy both healthy and tumor cells indiscriminately, MBTA therapy educates the immune system to selectively recognize and destroy tumor cells.
Proven Effectiveness
In mouse models, MBTA immunotherapy has shown extraordinary results:
- Up to 80% of mice were completely cured.
- The therapy prevented the growth of metastases.
- It created long-lasting immunity and resistance to recurrence.
These effects were confirmed in various types of cancer, including melanoma, sarcoma, colorectal cancer, pancreatic adenocarcinoma, pheochromocytoma, breast cancer, and glioblastoma.
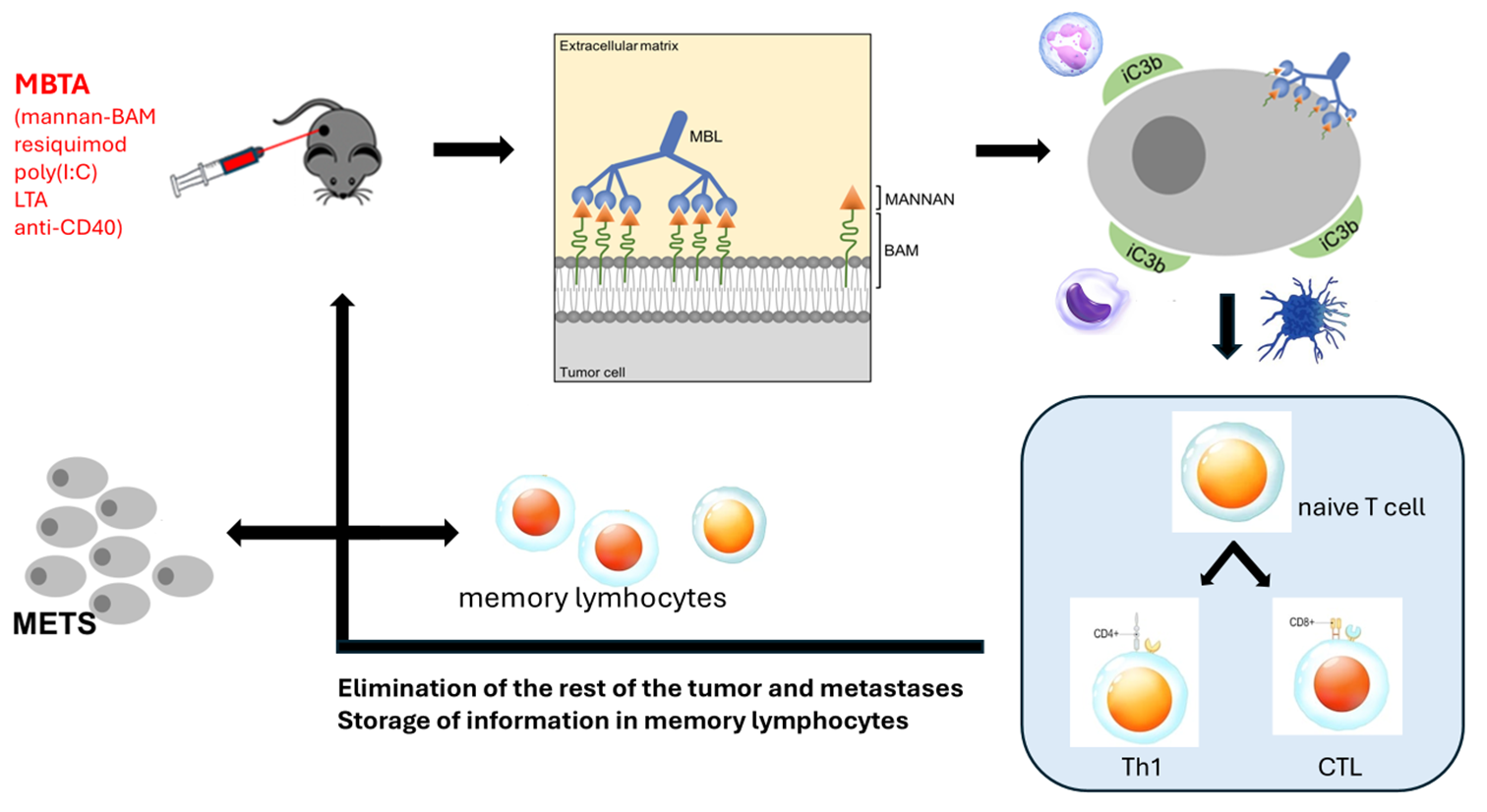
Diagram: Mechanism of MBTA Action
Mechanism of Action
The therapy is administered directly into the tumor (intratumoral application). This approach:
- Activates immune cells right in the tumor microenvironment.
- Helps the immune system recognize tumor antigens as dangerous.
- Initiates a cascade of immune reactions that target not only the treated tumor but also distant metastases.
MBTA acts like a “personalized cancer vaccine” that the body produces on its own – using the patient’s own tumor antigens.
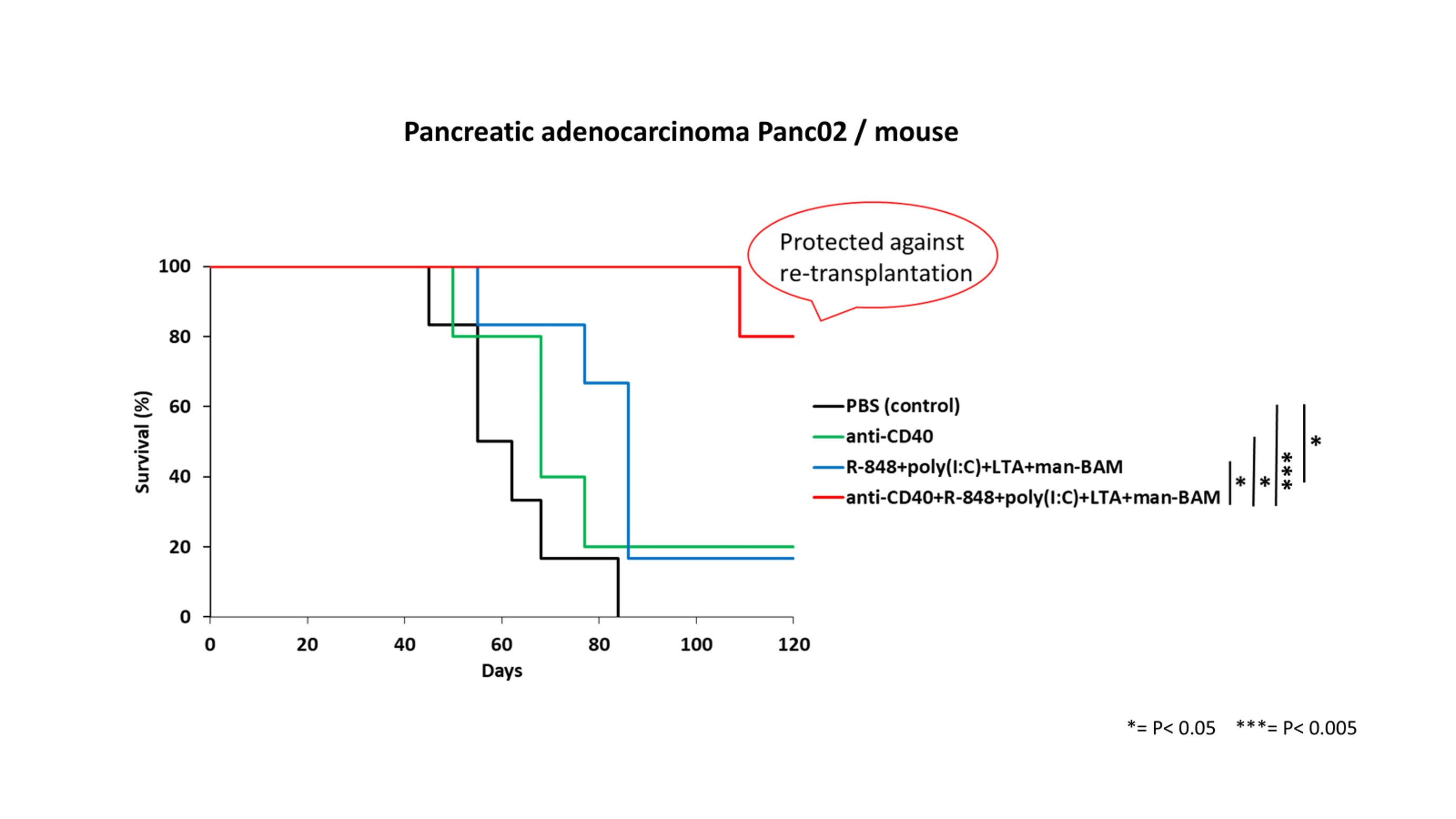
Graph: Survival of Treated Mice
Conclusion
MBTA therapy represents a new, scientifically grounded approach to cancer treatment with significant potential for clinical application.
We believe that this therapy – thanks to its unique mechanism and demonstrated effectiveness – may soon help cancer patients around the world.
Expert Opinions
“… it should be emphasized that the experimental tumor being tested is the very difficult-to-treat mouse melanoma B16-F10. Innate immunity is a phylogenetically strong mechanism that forms the basis for the more recently evolved adaptive immunity. Therefore, the scientific ambitions of Dr. Jan Zenka’s team should be considered not only modern but also as a promising immunotherapy approach potentially usable in future human patients. I am convinced that the experimental work of this team deserves comprehensive support.”
Prof. RNDr. Blanka Říhová, DrSc.
Chair of the Czech Immunological Society
Institute of Microbiology, Czech Academy of Sciences
“… I am familiar with Dr. Zenka’s work and I believe that his approach to immunotherapy is based on a solid foundation, with deep knowledge of both immunology and especially biochemical processes – knowledge many immunologists lack or disregard… The path from experiment to clinical application is long, financially demanding, and bound by many regulatory constraints. It depends on many factors not always related to the quality of the scientific work… In any case, I am convinced that Dr. Zenka’s research group deserves financial support for their research…”
Prof. MUDr. Jiřina Bartůňková, DrSc., MBA
Head of the Department of Immunology
2nd Faculty of Medicine, Charles University in Prague
“Dear Dr. Zenka:
Thank you for the opportunity to read the Ph.D. thesis of Veronika Caisova who is currently enrolled at your university.
My overall opinion of her body of work is that it satisfies the criteria for the award of a Ph.D. and would at almost all institutions in the United States.
The general thrust of her work, involving a novel approach to the immunotherapy of cancers, is a refreshing return to the very early work of W. B. Coley. His earlier work suggested that activation of the (probably innate) immune system in patients with cancer by bacterial products might lead to the eradication of some kinds of cancer. Ms. Caisova’s research greatly strengthens this concept and shows that in three different types of cancer the approach definitely has merit.
Ms. Caisova’s use of both phagocytosis stimulating ligands and activators of toll-like receptor agonists seems to be an effective approach. Although the major theme of her work is that this activation of the innate immune system might be beneficial, there is the added attraction that this activation might later lead to an adaptive immune response. This latter would provide a permanent cure for neoplastic disease.
So, my overall view of this work – and of Ms. Caisova’s clear grasp of cancer immunobiology – is highly favorable. My vote would approval of her thesis and the award of a doctoral degree.”
John W. Eaton, Ph.D., M.D.hc
James Graham Brown Professor of Cancer Biology
University of Louisville